What happens if you mix lithium and aluminum together?
Answers
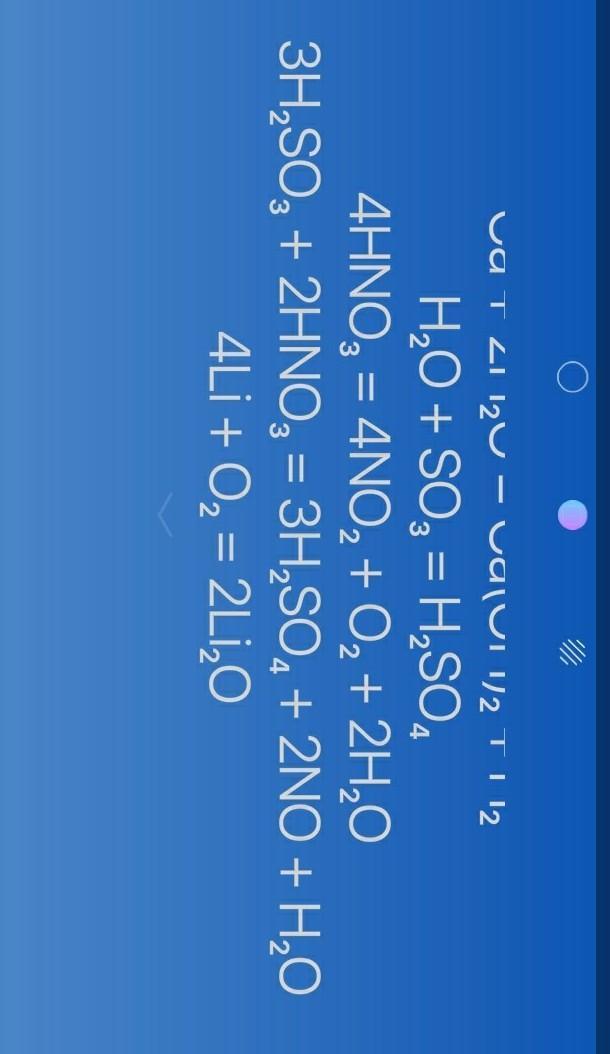
Answer:
hello
Explanation:
do you meant something like that

Related Questions
Why MgO will form after Mg reacting with CuO? (mcq)
Mg forms positive ions and O forms negative ions
Mg is more reactive than Cu
CuO have loose bonding
Answers
Answer:
Mg is more reactive than Cu
Explanation:
In the reaction between Mg and CuO to form MgO, MgO will form because Mg is more reactive than Cu.
This kind of reaction is called a single replacement or single displacement reaction.
The replacement of a metallic ion in solution by a metal atom higher in the activity series than the metal in solution falls into this category of reactions. The element Mg is higher than Cu in the activity series. The higher an element is in the activity series, the more reactive they are. Since Mg is more reactive, it will displace the Cu from CuO.Write the word equations for the following balanced chemical equations.
a. Zn + 2HCl → ZnCl2 + H2
b. 2503 2502 + O2
Answers
Answer:
21
Explanation:9+10
Name this element.
i need the answer like now
Answers
How many atoms are present in 7.50 mol of chlorine atoms? _
Answers
Answer:
4.52 x 10²⁴ atoms Cl
Explanation:
A mole is a name that means a certain number like a dozen means 12. 1 mole of chlorine atoms is 6.022 x 10²³ chlorine atoms. The unit conversion is 6.022 x 10²³/mol.
[tex]7.50molCl*\frac{6.022 x 10^{23} atomsCl}{1molCl} = 4.5165*10^{24} atomsCl[/tex]
Round to 4.52 x 10²⁴ atoms Cl for the correct number significant figures.
By what process do small molecules move into cells?
Answers
Answer:
small molecules move into the cell, Then it is by diffusion or facilliated diffusion.
A nuclear reaction, such as those which occur in a nuclear power plant, can be classified as what
type of reaction?
solar
chemical
exothermic
endothermic
Answers
What are the benefits and dangers of the Iron element in our daily life?
Answers
Refer to your periodic table:
Which of the following has the smallest atomic radius?
Bromine
Astatine
Iodine
Fluorine
Answers
iondine
Explanation:
bromine has 185pm, astatine haa 200, iondine has 140 and lastly fluorine has 147. so iondine has the smallest atomic radius
Plowing is an example of what Energy?
a. Kinetic Energy
b. Potencial Energy
Answers
Answer:
mechanical or kinetic eg hammer and nails
What is the relationship between the latitude and hours of daylight
Answers
Answer:
The tilt of the Earth's axis also defines the length of daylight.Daylight hours are shortest in each hemisphere winter. Between summer and winter
solistice the number of daylight hours decreases and the rate of decrease is larger the higher the latitude.The fewer sunlight hours the colder nights.
Answer question number 3
Answers
Answer:
A
Explanation:
A mixture is formed when two or more substances are physically mixed together. A compound is formed when two or more substances are chemically combined through a chemical reaction.
Your aquarium looks blurred and you wanted to clean it.How will you use recantation technique to separate water from the pebbles and other designs
Answers
Answer:
why do you not ask to your maam the answer
why is lithium used in the body?
Answers
Answer:
11
Explanation:
A roller coaster with a mass of 20kg sits at the top of a 30 m high hill. What is the Potential energy of the roller coaster?
(Formula: PE=mgh)
(g= 9.8m/s)
Answers
Answer:
The answer is 5880 JExplanation:
The potential energy of a body can be found by using the formula
PE = mgh
where
m is the mass
h is the height
g is the acceleration due to gravity which is 9.8 m/s²
We have
PE = 20 × 30 × 9.8
We have the final answer as
5880 JHope this helps you
6. A sheet of metal is 2 cm wide, 10 cm tall, and 15 cm long. It was 4 grams. What is the
density?
Answers
Answer:
0.013g/cm^3
Explanation:
density= mass÷volume
Answer:
0.01(3) g/cm3 (cm3 means centimeter cubed, (3) means the 3 goes on forever)
Explanation:
The volume of the sheet is 300 cm3, and dividing 4 grams by 300 cm3 gets you 0.01(3) g/cm3. Hope it helps!
N2+H2》NH3
is it balanced or unbalanced
Answers
N2 + 3H2 -> 2NH3
what unit of measure would i use to measure the width of my fingernail A millimeters
B centimeters C meters D kilometers
E grams
Answers
Because the width of fingernails are very small and millimeters is the smallest unit of measurement listed.
how many atoms can you fit on the head of a pin
Answers
Answer:
According to google, "About five million million hydrogen atoms could fit. Some factors would affect that number like the area of the head and the size of atoms (as well as attractions between atoms). Some atoms are larger than others." Is this accurate? I'm not sure. Good luck! :)
The density of air at STP is 1.285 g/L. Which of the following cannot be used to fill a balloon that will float in air at STP?
Answers
NO can't be used to fill a balloon
Further explanationConditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure). At STP, Vm is 22.4 liters/mol.
Answer options that need to be added :
a. Ne
b. NO
c. NH₃
d. CH₄
e. HF
will float in air ⇒ element or compound to fill the balloon, its density must be less than < 1.285 g/L
We can use the ideal gas formula ta find density :
[tex]\tt \rho=\dfrac{P.MW}{RT}[/tex]
Because at STP, then the constant value is
[tex]\tt \rho=\dfrac{1~atm\times MW}{0.08205\times 273.15~K}\\\\\rho=0.0446\times MW[/tex]
So that the density is determined from the MW(molecular weight) of each element or compound
a. NeAr = 20.1797 g/mol
[tex]\tt \rho=0.0446\times 20.1797=0.9~g/L[/tex]
b. NOMW=30.006 g/mol
[tex]\tt \rho=0.0446\times 30.006=\boxed{\bold{1.338~g/L}}[/tex]
c. NH₃MW=17.0306 g/mol
[tex]\tt \rho=0.0446\times 17.0306=0.760~g/L[/tex]
d. CH₄MW=16.04 g/mol
[tex]\tt \rho=0.0446\times 16.04=0.715~g/L[/tex]
e. HFMW=20.01 g/mol
[tex]\tt \rho=0.0446\times 20.01=0.892~g/L[/tex]
compound name of SO2
Answers
What is the effect of tap water, sea water, and rainwater on the rusting rate of iron?
(Please i need an answer π-π)
Answers
Answer:
The more acidic the solution the faster it rusts. More Na = more rust
Explanation:
An element, X, has 2 isotopes, X-32 with an abundance of 31.5% and X-35 with an abundance
of 68.5%. What is the average atomic mass of element X?
Answers
Answer:
amu = 34.055 or 34.1
Explanation:
to calculate the average mass, first convert the percentages into decimals. Then, calculate the mass numbers. To get this number, multiply the decimal by the mass number for each isotope and, then add them together.
( 0.315 x 32 ) + (0.685 x 35 ) = 34.055
A dull metal object has a density of 8.8 G/ML and a volume of 20 ML calculate the mass
Answers
Answer:
Mass = 0.000176 gram
Steps:
m = V × ρ
= 20 milliliter × 8.8 gram/cubic meter
= 2.0E-5 cubic meter × 8.8 gram/cubic meter
= 0.000176 gram
Explanation:
Hydrogen atoms are excited by a laser to the =4 state and then allowed to emit.
What is the maximum number of distinct emission spectral lines (lines of different wavelengths) that can be observed from this system? Calculate the wavelength of the 4⟶3 transition.
Answers
Answer:
1875 nm
Explanation:
Given the Rydberg formula for hydrogen: 1/λ = R(1/n₁² - 1/n₂²)
where R ≈ 1.097* 10^7 /m
Hence;
1/λ = 1.097* 107/m * (1/3² - 1/4²) = 5.3 * 10^5/m
λ = 1.875 * 10-6 m = 1875 nm
A piece of metal with a volume of 2.45 mL has a density of 4.255g/mL. What is the mass of the metal?
Answers
Answer:
The answer is 10.42 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
volume = 2.45 mL
density = 4.255g/mL
We have
mass = 2.45 × 4.255 = 10.42475
We have the final answer as
10.42 gHope this helps you
HELP!!!
write the scientific names of these ionic compounds
Answers
Answer:
• Copper chloride
• Copper (II) chloride
• Iron (II) sulfide
• Iron (III) sulfide
what happens to the molecules as water freezes PLS HELP THIS IS DO TODAY
Answers
During freezing, water molecules lose energy and do not vibrate or move around as vigorously. This allows more stable hydrogen-bonds to form between water molecules, as there is less energy to break the bonds. ... Thus water expands as it freezes, and ice floats atop water.
When water molecule freezes, the water molecules have slowed down enough that their attractions fixed them into fixed positions.
What do you mean by freezing ?The term freezing is defined as a phase transition where a liquid converted into a solid when its temperature is lowered below its freezing point.
The freezing point is the temperature at which a liquid turns solid. In the freezer, for example, water-filled ice cube trays.
Water molecules freeze in a hexagonal pattern, with molecules further apart than when the water was liquid. It is worth noting that the molecules in ice would be vibrating.
Thus, When water freezes, the water molecules slow down to the point where their attractions arrange them into fixed positions.
To learn more about the freezing, follow the link;
https://brainly.com/question/26230908
#SPJ2
A carpenter uses sandpaper on a piece of wood. Before, the wood felt rough. After, the wood felt smooth. Did the friction between the hand and the wood increase or decrease after using the sandpaper? Describe what the sandpaper did to the wood to make it smoother.
Answers
Answer:
Friction between the hand and the wood decreased.
Explanation:
The texture of the wood went from rough → smooth! This means friction between the hand and the wood was notably decreased.
According to this prompt, the carpenter used sandpaper against the wood. Sandpaper just so happens to be a very abrasive substance. The sandpaper polished and leveled out the wood which wore all the jutting bits away.- overall, making it much smoother and more pleasant to touch!
Hope I was of assistance! Have a nice day and Spread the Love! <3
Which group of atomic models is listed in historical order from the earliest to the most recent?
Answers
I saw this on a worksheet hope this helps!
The historical order of atomic models from the earliest to the most recent is as: Hard sphere model, electron shell model, and wave mechanical model. Therefore, option (A) is correct.
What is the atomic model?The atomic model can be described as the model used to describe the structure and composition of the atom. The atom as a primary component of matter has been under extensive study to understand the universe.
In the classical model of an atom, the atom is made of smaller particles with an electrical charge called electrons, protons, and neutrons. To understand the atomic models, it is important to understand how these particles make up the atom.
There are five principal atomic models that have been proposed over time, which are Dalton’s atomic model, Thomson’s atomic model, Rutherford’s atomic model, Bohr’s atomic model, and the quantum atomic model.
Learn more about the atomic model, here:
https://brainly.com/question/1596638
#SPJ5
Your question was incomple, most probably the complete question was,
Which group of atomic models is listed in historical order from the earliest to the most recent?
A) Hard sphere model, electron shell model, and wave mechanical model
B) Hard sphere model, wave mechanical model, and electron shell model
C) Electron shell model, Hard sphere model, and wave mechanical model
D) Electron shell model, wave mechanical model, and Hard sphere model
what makes a substance a molecule? it’s 7th grade science
Answers
Answer:
Molecular substances are made when two or more atoms join together by covalent bonds.
Explanation:
I hope this helps. I took the same class.