Plz answer quickly! :))
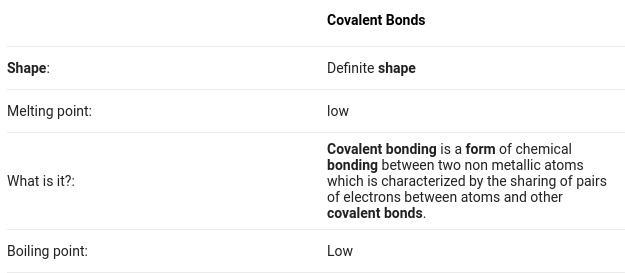
How are the shapes of covalent compounds made?
Answers
Hope this might help a little
Related Questions
Which models of the atom does the experimental evidence from Bohr's hydrogen experiment support? Explain why these models are compatible with the experimental results.
The models are Daltons, Thompsons, and Rutherfords
Answers
Answer:
Rutherfords
Explanation:
The model of the atom supported by Bohr's hydrogen experiment is the Rutherford's model of the atom.
Rutherford through his experiment on gold foil suggested the atomic model of the atom. The model posits that an atom has a small positively charged center(nucleus) where nearly all the mass is concentrated.
Surrounding the nucleus is the large space containing electrons. In the Bohr's model of the atom, he suggested that the extranuclear space of the atom is made up of electrons in specific spherical orbits around the nucleus.How are elements and compounds a part of our daily lives?
Answers
Answer:
Aluminium (A1) -Used in the production of duralumin alloy for use in the construction of aeroplane bodies. 3. Silicon (Si) -To make microchips 4. Sulphur (S) -To make matches, fireworks and for the manufacture of sulphuric acid 5.
Explanation:
Hope this helped!
Answer:
yes
Explanation:
we use different elements and compounds in our life ex salt and iron but most of the substances found in our environment are mixture btw scientists need to classified substances to work with our substances and to study pure substances hope it helps u
How much heat is required to raise the temperature of a 6.21 g sample of iron from 25.0 oC to 79.8 oC?
Answers
Answer:
151.1J
Explanation:
Given parameters:
Mass of iron = 6.21g
Initial temperature of iron = 25°C
Final temperature of iron = 79.8°C
Unknown:
Amount of heat = ?
Solution:
The amount of heat require to cause this temperature can be determined using the expression below;
H = m c (T₂ - T₁)
H is the amount of heat
m is the mass
c is the specific heat capacity
T is the temperature
Specific heat capacity of iron 0.444J/g°C
Insert the parameters and solve;
H = 6.21 x 0.444 x (79.8 - 25)
H = 151.1J
What are the coefficients that will balance the skeleton equation below?
___GaF3 +___KCN rightwards arrow___Ga(CN)3 + ___KF
A.
1, 3, 1, 3
B.
3, 1, 3, 1
C.
1, 1, 1, 3
D.
1, 3, 3, 1
1 points
QUESTION 6
The previous question is an example of what type of chemical equation?
A.
Synthesis
B.
Decomposition
C.
Single Re-Placement
D.
Double Re-Placement
Answers
1, 3, 1, 3
Explanation:
Ga - one on both sides
F - three on both sides
CN - three on both sides
K - three on both sides
But remember that CN was being multiplied by 3 on the right side already, meaning GA(CN)3 didn't mean a coefficient.
Answer:
Double re-placement
The two metals switched dance partners, that means it's a double.
10 g of copper is heated and the mass increases to 12.5 g. What is the mass of the copper in the new substances?
Answers
Answer:
10g
Explanation:
The amount of copper in the heated mass of the new substance produced is still 10g because in any chemical action, mass is always conserved.
According to the law of conservation of matter "matter is neither created nor destroyed in a chemical reaction". The change in mass is only due to oxygen gas that combines with the new copper formed or other substances that joined the mass. The substance will still have the same amount of copper as the original one. Since we started with 10g of copper, we end with 10g of copper.Please help i will give brainliest
Answers
PLEASE HELP ME !!!!!!!
Answers
Answer:
it would be 5,045
Explanation:
because it is closer to 5,000. pls correct me if wrong
Question 4 of 10
What is the molarity of a solution of 3 moles (mol) of FeBr3 in 1/2 L water?
A.
3 mol/2L
B.
2L/3 mol
C.
0.5L/3 mol
D.
3 mol/0.5 L
SUBMIT
Answers
Answer:
B
Explanation:
Answer:
D. 3 mol/0.5 L
Explanation:
Just took the quiz
Metals react by losing valence electrons. Which of the alkali metals loses its valence electrons most easily? Explain.
Answers
Answer:
lol you have an aot profile pic
Question 2 (1 point)
Which of the following is the main reason the transition metals are able to exist in different forms?
а.
predictability in ability to bond
B.
unfilled d orbitals
C.
magnetic properties
D.
predictability in number of valence electrons
Answers
Answer:
B. Unfilled d orbitals
Explanation:
which statement below best describes the movement of particles in a gas?
A- the particles vibrate in a fixed position
B-the particles slide easily past one another
C- the particles move freely and are well separated
D- the particles move freely with little free space between the particles.
Answers
Answer:
c the particles are spaced out and move freely
Explanation:
Answer:
C). The particles move freely and are well separated
Explanation:
8. Consider the element Scandium, atomic # 21.
(a) If the electronic configuration of the element were constructed "from scratch", into which
orbital (and into which shell) would the final electron be placed? (1)
(b) When scandium forms an ion with a charge of +1, from which orbital (and from which
shell) would the electron be removed? (1)
Answers
Explanation:
Scandium has atomic number of 21. This means that in it's neutral state its going to have 21 electrons.
a) The full electronic configuration is given as;
1s2 2s2 2p6 3s2 3p6 3d1 4s2
The final electron is placed in the d orbital. The shell is 3d
(b) When scandium has a charge if +1, it has lost an electron. The total number of electrons would now be 21-1 = 20
The electronic configuration would be given as;
1s2 2s2 2p6 3s2 3p6 4s2
The electron in the 3d orbital would be removed.
One isotope of oxygen differs from another isotope of oxygen in *
Answers
Each isotope of Oxygen has a different number of neutrons
Further explanationThe elements in nature have several types of isotopes
Atomic mass is the average atomic mass of all its isotopes
Isotopes are atoms has the same number of protons but has a different number of neutrons.
So Isotopes are elements that have the same Atomic Number (Proton)
Some of the isotopes of oxygen are:
[tex]\tt _8^{16}O,_8^{17}O,_8^{18}O[/tex]
Each isotope has 8 protons and 8 electrons but has a different number of neutrons
For O-16: number of neutrons = 16-8 = 8
For O-17: number of neutrons = 17-8 = 9
For O-18: number of neutrons = 18-8 = 10
In an experiment conducted between oxygen and chlorine gas it was found that oxygen gas spread much faster than chlorine. What is the explanation for this observation?
Answers
Answer:
Because oxygen is lighter
Explanation:
how is a solute and a solvent different
Answers
I WILL GIVE YOU BRAINLIEST HELP HELP HELP THE SUBJECT IS ACTUALLY SCIENCE What factor is deer in an ecosystem? Abiotic or Biotic? the subject is actually science
Answers
Answer: the answer is biotic because dear are a living factor
Explanation:
a solid sample of oil until it turns an iquid. which property of the oil will decrase?
Answers
Answer:
Density of the oil
Explanation:
As the oil sample is warmed and it turns liquid, the density of oil will decrease due to increase in volume of the oil.
Warming of the oil increases the volume of oil as it melts. The once solid oil becomes more mobile and takes up more space. Although the mass is the same. Since volume increases, the density of the oil will reduce because, density is the mass per unit volume of a substance.Which equation represents a double replacement reaction?
A) 2 Na + 2 H2O → 2 NaOH + H2
B) CaCO3 → CaO + CO2
C) LiOH + HCl → LiCl + H2O
D) CH4 + 2 O2 → CO2 + 2 H2O
Answers
Answer:
C) LiOH + HCl → LiCl + H₂O
General Formulas and Concepts:
Chemistry - Reactions
Synthesis Reactions: A + B → AB Decomposition Reactions: AB → A + B Single-Replacement Reactions: A + BC → AB + C Double-Replacement Reactions: AB + CD → AD + BCExplanation:
Step 1: Define
RxN A: 2Na + 2H₂O → 2NaOH + H₂
RxN B: CaCO₃ → CaO + CO₂
RxN C: LiOH + HCl → LiCl + H₂O
RxN D: CH₄ + 2O₂ → CO₂ + 2H₂O
Step 2: Identify
RxN A: Single Replacement Reaction
RxN B: Decomposition Reaction
RxN C: Double Replacement Reaction
RxN D: Combustion Reaction
Helpppppp i hate chemistry
I will give brainiest who helps me with the correct answer
I promise
5. Name the compound which contain Strontium, Fluorine, and Oxygen.
Options:
Strontium fluorate
Strontium fluoride
Strontium chlorate
Strontium fluorine
Answers
2. What properties do all acids have?
3. Why should you never use taste as a method for testing an unknown substance?
4. What is used to detect the presence of acids?
10. What can you use to detect the presence of a base?
11. What are some common bases that we use every day?
12. How does an antacid help reduce heartburn?
13. What happens when acid and base are mixed
14. What is used to determine the pH of a substance?
15. Describe how the pH scale works. In other words, what is it actually measuring?
17. Why is pH important to our environment?
18. Describe how salt is formed.
Pls help!!!!!
Answers
3. You should NEVER taste unknown chemicals because many are poisonous and can destroy body tissue.
4. Most often dyes are used to detect acids. The dyes will turn a certain color which indicates the presence of an acid.
10. You can use litmus to detect wether a solution is a base or an acid.
11. The most common bases I can think of would be: baking soda, laundry detergent, or soaps.
12. Antacids work by neutralizing the acids in your stomach.
13. When an acid and base are mixed together, they produce a salt.
14. Normally pH meters are used, but you can also calculate the pH using math. (yuck)
15. The pH scale measures how acidic or basic a substance is. It ranges from 0-14, with 7 being neutral.
17. pH is very important to the environment as it helps us measure and monitor for safe water conditions. A rise or fall from the average water pH level can indicate chemical pollution.
18. Salt is formed when an acid and base react with each other. It then has a cation of the base, and anion of the acid.
I hope those helps!! (:
Why should a hypothesis be testable?
A. It must be testable in order to be found true or false.
B. It must be testable for the outcome to be predictable.
C. It must be testable for other scientists to consider it reliable.
D. It must be testable if it is to become a scientific theory.
Answers
Calculate how much Carbon Dioxide IN GRAMS needs to be absorbed to
release 500g of oxygen during photosynthesis.
Answers
Answer:
687.5 g of CO2
Explanation:
We'll begin by writing the balanced equation for photosynthesis. This is illustrated below:
6CO2 + 6H2O —> C6H12O6 + 6O2
Next, we shall determine the mass of CO2 absorbed and the mass of O2 released from the balanced equation. This is illustrated below:
Mass of CO2 = 12 + (2 × 16) = 12 + 32
= 44 g/mol
Mass of CO2 from the balanced equation = 6 × 44 = 264 g
Molar mass of O2 = 2 × 16 = 32 g/mol
Mass of O2 from the balanced equation = 6 × 32 = 192 g
Summary:
From the balanced equation above,
264 g of CO2 were absorbed to release 192 g of O2.
Finally, we shall determine the mass of CO2 absorbed to release 500 g of O2. This can be obtained as follow:
From the balanced equation above,
264 g of CO2 were absorbed to release 192 g of O2.
Therefore, Xg of CO2 will be absorbed to release 500 g of O2 i.e
Xg of CO2 = (264 × 500)/192
Xg of CO2 = 687.5 g
Thus, 687.5 g of CO2 will be absorbed to release 500 g of O2.
CH4+O2--->CO2+2H2O2
Write a few paragraphs predicting the products of the chemical reaction and explaining why you made this prediction. Your document should:
i. identify the products of the chemical reaction.
ii. explain why these are the products based on trends in the periodic table (the number of valence electrons, electronegativity, etc.).
iii. identify the types of bonds in the reactants and products.
iv. identify the number of each type of bond in the reactants and products.
v. identify the type of reaction.
Answers
Riley Greene
Initial prediction : CH_4+O_2 transfers to CO_2+H_2O
Initial Explanation :
The products of this reaction are CO_2 and H_2O. These elements are Carbon Dioxide and Water. Atom's chemical properties are determined by the number of electrons in its outermost shells. Based on the number of electrons in the shells, it decides the reactivity of atoms. Atoms with the same number of electrons in the outer shells have similar chemical properties. If atoms have 1,2 or 3 valence electrons they react by giving out those electrons to become stable. If atoms have 6 or 7 electrons in the outer shell they react by taking in electrons to complete its outer shell.
So Methane has 8 valence electrons while Oxygen has 6. One molecule of methane combined with two oxygen molecules, react to form a carbon dioxide molecule, and two water molecules usually given off as steam or water vapor during the reaction and energy.
In the reaction, the bonds in the methane and oxygen come apart, the atoms rearrange and then re-bond to form water and carbon dioxide.The reactants are on the left side of the equation and the products are on the right. In the reaction, the bonds in the methane and oxygen come apart, the atoms rearrange and then re-bond to form water and carbon dioxide. No new atoms are created and no atoms are destroyed. This reaction is a combustion reaction.
Dot diagram Attached....
Revised prediction : CH_4+O_2 transfers to CO_2+H_2O
Revised Explanation : I was correct initially so I put the same explanation.
The products of this reaction are CO_2 and H_2O. These elements are Carbon Dioxide and Water. Atom's chemical properties are determined by the number of electrons in its outermost shells. Based on the number of electrons in the shells, it decides the reactivity of atoms. Atoms with the same number of electrons in the outer shells have similar chemical properties. If atoms have 1,2 or 3 valence electrons they react by giving out those electrons to become stable. If atoms have 6 or 7 electrons in the outer shell they react by taking in electrons to complete its outer shell.
So Methane has 8 valence electrons while Oxygen has 6. One molecule of methane combined with two oxygen molecules, react to form a carbon dioxide molecule, and two water molecules usually given off as steam or water vapor during the reaction and energy.
In the reaction, the bonds in the methane and oxygen come apart, the atoms rearrange and then re-bond to form water and carbon dioxide.The reactants are on the left side of the equation and the products are on the right. In the reaction, the bonds in the methane and oxygen come apart, the atoms rearrange and then re-bond to form water and carbon dioxide. No new atoms are created and no atoms are destroyed. This reaction is a combustion reaction.
The reaction as shown is a combustion reaction.
The reaction; CH4 + O2 ---> CO2 + 2H2O2 is a combustion reaction because methane reacts with oxygen to yield carbon dioxide and water. Carbon is always tetravalent in its compounds hence there are four carbon oxygen bonds in the product. Lets recall that the bonds in CH4 re nonpolar because of the minimal electronegativity difference between carbon and hydrogen. Even though the bond in CO2 is polar but the eneitire molecule is nonpolar because the dipoles cancel out. Water is a polar molecule.
Hydrogen forms only one bond hence in water molecule, each hydrogen atom is bonded to a central oxygen atom. All the bonds in water and carbon dioxide as well as in methane and oxygen are covalent.
Learn more: https://brainly.com/question/14283892
Which of the following equations is not balanced?
Answers
Explanation:
i think the answer is first and last
last one is balanced!!
When the following equation is balanced, what is the coefficiant for Br?
___I2 +___NaBr rightwards arrow___NaI +_?_Br2
A.
6
B.
3
C.
1
D.
2
1 points
QUESTION 10
The previous question is an example of what type of chemical reaction?
A.
Synthesis
B.
Decomposition
C.
Single Re-Placement
D.
Double Re-Placement
Answers
Answer:
1. A 2. C
Explanation:
because it just is
Calculate the percent composition of C6H12O6
Answers
Answer:
Did you just let your cat walk on your keyboard? XD
Could you explain this a bit better please?
I'll answer the question in the replies of this comment if you can give me a bit more information.
Explanation:
May I have brainliest please? :)
Write the electron configuration for the electrons identified by the following quantum numbers:
a) 2, 1, 0, -½
b) 4, 2, 0, -½
c) 5, 3, 2, -½
Thanks!
Answers
Answer:
see the answer at the pic
The electron configuration for the electrons as listed are:
a) 1s² 2s² 2p⁵ = 9X
b) 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d⁸ = 46 X
c) 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 5p⁶ 6s² 4f¹⁴ 5d¹⁰ 6p⁶ 7s² 5f¹⁴
Using the quantum numbers of the identified electrons to derive the electron configuration ( the representation of the distribution of electrons in the orbit of an atom ) electron configuration of electrons can be calculated using the formula :2n².
where n = orbit number and the various energy levels are represented as 1,2,3,4... while shells of the an atom is written as K,L,M,N
hence the electron configuration of the electrons identified by the quantum numbers are as written above.
Learn more : https://brainly.com/question/20974986
The growth of a plant toward light is called _____.
phototropism
tropism
geotropism
Answers
It's called phototropism
Answer:
The answer is (Phototropism)
3. Does the siren of a moving police car seem to change pitch to the
police officers inside the car? Why or why not?
Answers
Answer:
As the police car moves towards them, the pitch of the police car's siren goes up because the sound waves get closer together in front of the police car. When the police car moves away from the person, the pitch goes down because the waves spread out behind the police and that makes the frequency go down.
Explanation:
Below is a phase diagram for Carbon Dioxide. Use this diagram to answer the following question.
In this example we will start the Carbon Dioxide out at a temperature of -20oC and 1 atm of pressure. If the pressure was raised to 100 atm of pressure while still staying at -20oC, what phases would the Carbon Dioxide change between?
Answers
Vapor to liquid is the same as saying gas to liquid, which I believe to be the correct answer.